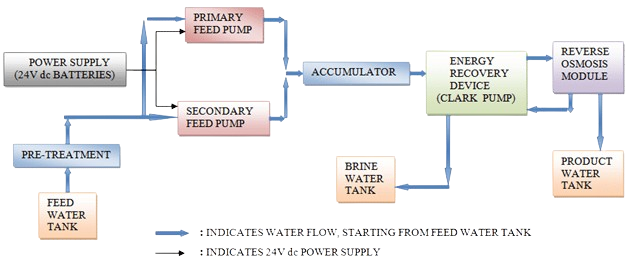
Reverse osmosis (RO) is a water purification process that uses a semi-permeable membrane to separate water molecules from other substances. RO applies pressure to overcome osmotic pressure that favors even distributions. RO can remove dissolved or suspended chemical species as well as biological substances (principally bacteria), and is used in industrial processes and the production of potable water. RO retains the solute on the pressurized side of the membrane and the purified solvent passes to the other side. It relies on the relative sizes of the various molecules to decide what passes through.
"Selective" membranes reject large molecules, while accepting smaller molecules (such as solvent molecules, e.g., water).[1] RO is most commonly known for its use in drinking water purification from seawater, removing the salt and other effluent materials from the water molecules.A process of osmosis through semi-permeable membranes was first observed in 1748 by Jean-Antoine Nollet. For the following 200 years, osmosis was only a laboratory phenomenon.
In 1950, the University of California at Los Angeles (UCLA) first investigated osmotic desalination. Researchers at both UCLA and University of Florida desalinated seawater in the mid-1950s, but the flux was too low to be commercially viable.[4] Sidney Loeb at UCLA and Srinivasa Sourirajan[5] at the National Research Council of Canada, Ottawa, found techniques for making asymmetric membranes characterized by an effectively thin "skin" layer supported atop a highly porous and much thicker substrate region. John Cadotte, of Filmtec corporation.

 By eliminating impurities and dissolved solids, reverse osmosis can significantly improve the taste, odor, and overall quality of water.
By eliminating impurities and dissolved solids, reverse osmosis can significantly improve the taste, odor, and overall quality of water. RO systems effectively remove a wide range of contaminants from water, including dissolved salts, heavy metals, minerals, organic compounds, bacteria, viruses, and other impurities.
RO systems effectively remove a wide range of contaminants from water, including dissolved salts, heavy metals, minerals, organic compounds, bacteria, viruses, and other impurities. RO systems offer a convenient and reliable way to access purified water at home, in offices, and in other settings.
RO systems offer a convenient and reliable way to access purified water at home, in offices, and in other settings. RO systems provide access to clean, safe drinking water, which is essential for maintaining good health.
RO systems provide access to clean, safe drinking water, which is essential for maintaining good health.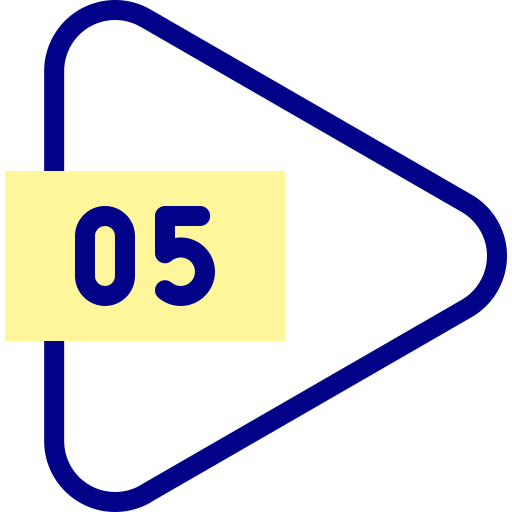 Using reverse osmosis to purify water can have positive environmental implications.
Using reverse osmosis to purify water can have positive environmental implications.